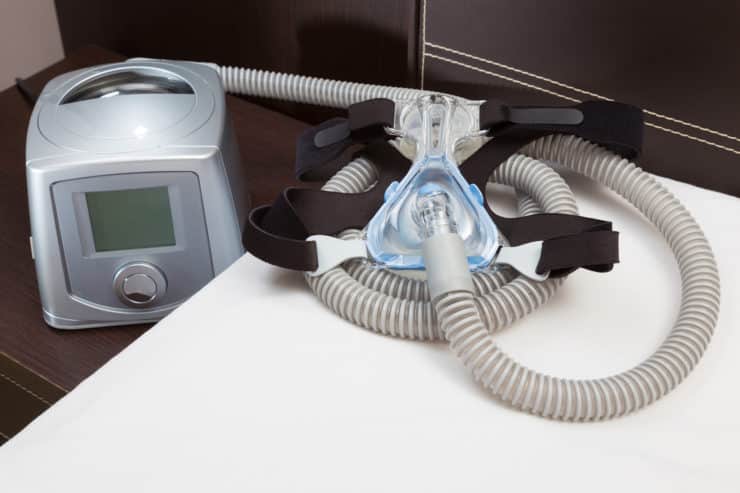
Philips CPAP Lung Injury Lawsuit
Philips is facing hundreds of CPAP lawsuits for injuries or deaths due to degradation of the foam liner and outgassing of toxic chemicals in their breathing machines. In addition, the company may be responsible for the replacement of millions of devices which may place users at increased risk of injury or illness.
As of October 2023, over 700 Philips CPAP Lawsuits have been consolidated into multidistrict litigation (MDL) in the U.S. District Court for the Western District of Pennsylvania. Bellwether trials are expected to begin in 2025. Other cases have been filed in both federal and state courts and more are expected to be filed in the near future.
In September 2023, Seeger Weiss partner Chris Seeger announced an uncapped settlement with Philips to resolve economic loss claims. The settlement provides a minimum of $479 million for people and payors who were financially harmed by the recall. Separately, Philips has also been ordered to pay $62 million to resolve allegations by the Securities and Exchange Commission (SEC).
Illnesses that have been reported include:
- Respiratory:
- New Onset COPD
- New Onset Asthma
- Pulmonary fibrosis
- Sarcoidosis
- Pneumonitis
- ARDS
- RADS (Reactive Airway Disease)
- Bronchiectasis
- Bronchiolitis Obliterans
- Bronchitis
- Emphysema
- Cancer:
- Lung Cancer
- Nasal Cancer
- Soft Palate Cancer
- Sinus Cancer
- Throat Cancer
- Tonsil Cancer
People who were injured after using a Philips Bi-Level Positive Airway Pressure (Bi-Level PAP), Continuous Positive Airway Pressure (CPAP) or mechanical ventilator device or who have used one of these devices may be eligible for compensation.
Philips CPAP Breathing Machines Recalled Over Potential Lung Injury
Millions of Philips Respironics breathing machines have been recalled due to a potential risk of lung injury. Phillips has issued a recall for Philips Bi-Level Positive Airway Pressure (Bi-Level PAP), Continuous Positive Airway Pressure (CPAP), and mechanical ventilator devices over concerns that the polyester-based polyurethane foam liners may disintegrate or outgas. This may potentially result in inhalation of contaminants and/or toxic chemicals, increasing the risk of cancer or serious respiratory illness.
The FDA has received over 98,000 adverse event reports relating to the use of the recalled Philips CPAP devices, including at least 346 deaths. Philips may be facing numerous Philips CPAP recall lawsuits filed by people who experienced injury or cancer after using a Philips sleep apnea CPAP, Bi-level PAP, or mechanical ventilator.
Philips CPAP Recall Upgraded to Class 1
Philips Respironics issued an April 2021 voluntary recall of some Philips Sleep Apnea Devices. The recall notification was amended as a safety notification in June 2021 and further in July 2021 to a Class 1 recall, indicating that the product may cause serious injury or death. In January 2022, the FDA announced that additional ventilators and ventilator repair kits were added to the recall list.
The recall involves millions of Philips sleep apnea and ventilator machines, most of which are of the DreamStation, first-generation production line and are within the 5-year service life. The recall has been issued for machines which contain a sound-abatement foam comprised of polyester-based polyurethane (PE-PUR) which may degrade to release particles and chemicals that may be inhaled.
Philips estimates that up to 4 million devices may be affected and has constrained the recall to the U.S. market. As of July 2023, two years after the recall, hundreds of thousands of patients had not received replacement devices as a result of a shortage. The company has stated that the shortage could last until the end of 2023.
Recalled Philips Devices Include:
CPAP and BiLevel PAP
Continuous Ventilator, Non-life Supporting
- DreamStation ASV
- DreamStation ST, AVAPS
- SystemOne ASV4
- C Series ASV, S/T, AVAPS
- OmniLab Advanced Plus In-Lab Titration Device
Non-continuous Ventilator
- SystemOne Q series
- DreamStation CPAP, Auto CPAP, BiPAP
- DreamStation Go CPAP, APAP
- Dorma 400, 500 CPAP
- REMStar SE AutoCPAP
Continuous Ventilator, Minimum Ventilatory Support, Facility Use
- E30 (Under Emergency Use Authorization)
Mechanical Ventilators
Continuous Ventilator
- Trilogy 100Ventilator
- Trilogy 200Ventilator
- Garbin Plus, Aeris, LifeVentVentilator
Continuous Ventilator, Minimum Ventilatory Support, Facility Use
- A-Series BiPAP V30 AutoVentilator
Repair Kit
- Trilogy Evo repair kit
Philips Recall Recommendations and Concerns
The FDA safety and recall notification for Philips breathing machines includes instructions on when to discontinue using the devices. People using non-life supporting devices such as CPAP are advised to discontinue using their machines and consult with their physician. Those using life-supporting ventilators should not stop using them but should immediately seek medical assistance.
In September 2021, Philips issued notification that it had received approval from the FDA to replace the troublesome foam lining or replace devices with new models. On November 12, 2021, however, the FDA issued another notification indicating that silicone foam used in replacement devices may also pose a safety risk. The Agency is requiring the company to submit independent testing of the replacement phone.
The FDA has also expressed concern that the problems may be more severe than has been recently represented by Philips. Some documents suggest that testing may have only been done on new devices, when many of the Philips CPAP devices in use are actually refurbished devices which may be subject to greater degradation.
Though the company has intended to replace recalled devices, many patients have had trouble obtaining replacements due to a shortage created by the recall and other issues including supply chain issues during COVID-19. Others have complained of trouble with a burning smell in the replacement devices.
Sound-reducing Foam Liner May Be to Blame
The June 2021 voluntary Philips Respironics CPAP and ventilator recall was issued due to a possibility of increased risk of airway contamination or inhalation of particles or chemicals. The recall was later upgrade to an FDA Class 1 recall, due to serious risk of injury or death. The effects on the user may include lung injury, chemical exposure, and an increase in cancer risk.
Risks of lung injury and cancer are related to polyester-based polyurethane (PE-PUR) foam which is a sound-abatement foam component of certain models of the devices. PE-PUR foam may degrade into particles, entering into the device air pathway, to be inhaled or ingested. In addition, the foam may also be prone to “off-gas” chemicals which may be toxic.
Devices which are more than three years old are more likely to have foam degradation. Philips has stated that degradation and outgassing may also be worsened by the use of unapproved cleaning methods including high heat, high humidity, and ozone cleaning environments.
Once company, SoClean, has disputed claims by Philips that ozone cleaning methods are to blame and has filed a lawsuit against Philips for false and misleading statements.
Philips Delayed Action on Degrading Foam in CPAP Devices
According to discovery documents for Philips CPAP Recall Lawsuits, internal communications show that the company may have known about the potential risks of the devices as early as 2015. In April 2018, three years before the first recall, a Philips engineering employee contacted the supplier of the foam used in the devices. Emails and customer reports show concerns over foam degradation and shedding, and the potential for inhalation of shed particles.
Philips has also been accused of making misleading statements about another device maker, SoClean over its ozone cleaning devices used on CPAP machines. SoClean filed a federal lawsuit against Philips in October 2021. The lawsuit claims that Philips misled the public by placing blame for foam degradation on SoClean’s ozone cleaning product to distract from flaws inherent in the Philips breathing machines. The complaint was amended in December 2021, to include information from FDA inspection of Philips manufacturing which showed that Philips had known about the foam degradation issues.
Exposure to Degradation of Philips Breathing Machines
The potential degradation of PE-PUR foam in Philips CPAP breathing devices and ventilators may lead to inhalation of degradation products, particles, and toxic chemicals. This may increase the chance of lung injury and may also increase the risk of cancer.
Symptoms of exposure may include:
- Headache
- Throat or mucous membrane irritation
- Coughing
- Lung inflammation
- Chest pressure
- Sinus infection
- Nausea and vomiting
- Carcinogenic effects
People who experience symptoms should seek medical assistance. Those who are using a life-supporting ventilator should not discontinue using the device but should seek immediate medical attention.