Case Info
Transvaginal Mesh Implants as Part of Pelvic Repair Systems
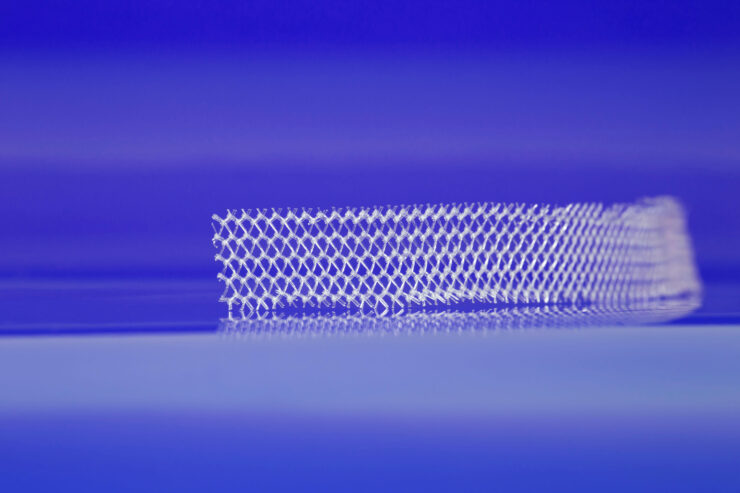
Transvaginal mesh is a type of surgical mesh that was surgically implanted as part of a pelvic repair system. It was used to support and stabilize weakened pelvic and bladder tissues in women with conditions such as stress urinary incontinence (SUI) and pelvic organ prolapse (POP). Rather than implantation through an open abdominal surgery, transvaginal mesh was implanted through the vaginal opening or “transvaginally”.
Transvaginal Mesh Injuries
Transvaginal mesh has caused injury to hundreds of thousands of women and resulted in multiple deaths. Studies have shown that as many as 10 percent of women who received a transvaginal mesh product may have serious complications.
Side effects of transvaginal mesh may include:
- Pain or discomfort of the vagina or in the surrounding pelvic region
- Nerve damage including pain or loss of sensation in the vagina or surrounding pelvic region
- Vaginal scarring
- Vaginal shrinkage
- Pain during intercourse
- Recurrence of POP or SUI
- New occurrence of SUI
- Chronic urinary tract infection
- Increased risk of infection
- Excessive vaginal bleeding
- Vaginal wall erosion
- Vaginal or other organ perforation
- Need for revision surgery
- Emotional trauma
These side effects may evolve into serious adverse events which require extensive medical treatment and may be permanent or life-threatening.
In 2019, under the guidance of the U.S. Food and Drug Administration (FDA), transvaginal mesh was discontinued and is no longer sold in the U.S. It has also been banned in many other countries.
Lawsuits Against Transvaginal Mesh Manufacturers
Over 100,000 transvaginal mesh lawsuits were filed by women and loved ones of those who had been harmed against medical device makers including:
- American Medical Systems, Inc. / Endo International
- Boston Scientific Corp.
- R. Bard, Inc.
- Coloplast Corp.
- Cook Medical, Inc.
- Ethicon, Inc. / Johnson & Johnson
- Neomedic
Though a few lawsuits remain in state and local courts, most transvaginal mesh lawsuits were consolidated into one of seven separate federal multidistrict litigation (MDL) cases. The majority of these cases have been resolved, awarding victims settlements that ranged from tens of thousands to millions of dollars.
Transvaginal Mesh Settlements:
American Medical Systems, Inc. (AMS): By 2014, AMS had already agreed to pay $830 million for approximately 20,000 claims. In 2017, AMS’s parent company, Endo International, also set aside $775 million for an additional 22,000 claims. Estimates show that the company has paid approximately $2.6 billion for transvaginal mesh lawsuits over a period of more than 10 years.
Boston Scientific Corp.: In April 2015, Boston Scientific agreed to pay approximately $119 million to settle nearly 3,000 transvaginal mesh lawsuits and agreed to resolve another 50,000 potential claims. The company has also paid nearly $200 million to state agencies and agreed to a $105 million settlement in Australia.
Coloplast Corp.: In 2014, Coloplast settled approximately 400 transvaginal mesh lawsuits for nearly $16 million.
C.R. Bard, Inc.: After losing both federal and state lawsuits, Bard settled approximately 500 lawsuits for $21 million and later settled another 3,000 cases for about $200 million.
Ethicon, Inc.: After losing several bellwether trials, including an $11 million verdict for the plaintiff, Ethicon settled most cases for undisclosed amounts. The company later paid millions of dollars to multiple state governments over injuries caused by transvaginal mesh.