Case Info
Fosamax (alendronate) is a bisphosphonate medication used to treat and prevent osteoporosis in men and women and to treat bone loss from other medical conditions including Paget’s disease and glucocorticoid use.
Though Fosamax is intended to reverse bone loss, improve bone density, and protect against osteoporosis, it may have increased the chance for serious injuries including:
- Osteonecrosis of the jaw
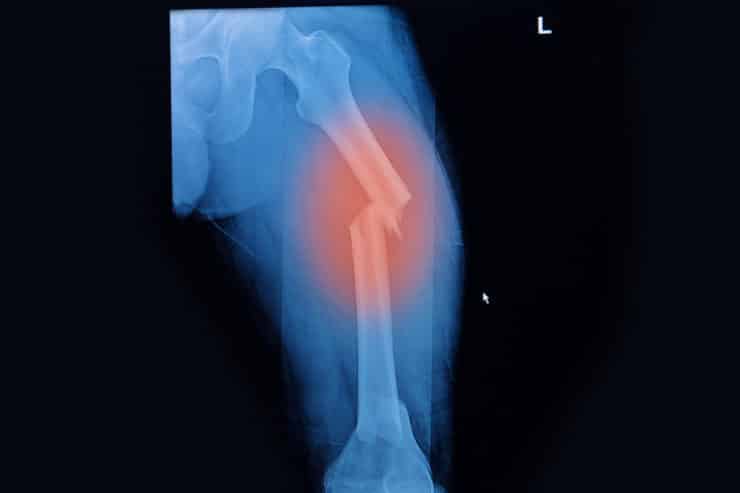
- Femur fracture
- Other bone injuries
Pharmaceutical manufacturer, Merck, has faced thousands of lawsuits filed by people who have suffered jaw damage, bone fracture or osteonecrosis after taking Fosamax.
Drug Injury: Fosamax
Fosamax (alendronate) is a member of the bisphosphonate class of medications, which works to prevent or reverse bone porosity or loss of bone density. It works by inhibiting activity of the osteoclasts, which are cells that work to remove minerals such as calcium from the bone tissue so that it can be used in other areas of the body. Fosamax’s interference with osteoclasts can reduce bone turnover, and ultimately, help to prevent or treat bone loss.
| Brand Name | Fosamax |
| Generic Name | alendronate |
| Classification | bisphosphonate |
| Manufacturer | Merck & Co |
| Dosage form(s) |
|
| Normal dosage | Treatment of osteoporosis in postmenopausal women
Prevention of osteoporosis in postmenopausal women
Treatment of osteoporosis in men
Treatment of glucocorticoid-induced osteoporosis
Treatment of Paget’s Disease
|
Fosamax Femur Fracture
Fosamax (alendronate), a popular drug in the bisphosphonate class of medications, is used for the treatment of osteoporosis and other bone disorders in both men and women. Though the drug is intended to strengthen bones and reduce bone porosity leading to degradation and fracture, strong evidence has shown long-term use of Fosamax may have the opposite effect in some patients.
Some evidence shows the drug may contribute to osteonecrosis (bone death) involving the jawbone. And many women who have taken the drug for five years or more have experienced atypical femur fractures during normal everyday activities without apparent cause. Patients who are subject to loss of bone density and are given Fosamax may be unaware of the side effects, which can lead to femur fracture, osteonecrosis, or other bone loss.
Fosamax Osteonecrosis of the Jaw
Fosamax and other bisphosphonates may increase the chance for a severe bone disorder known as osteonecrosis of the jaw (ONJ). Osteonecrosis occurs when the bone tissue begins to die due to an inability of the body to continue to supply blood and oxygen to the area. When osteonecrosis occurs in the jaw, sections of the bone or jaw may require removal.
Fosamax may also cause other side effects including:
- Abdominal pain
- Nausea
- Constipation
- Headache
- Rash
- Atrial fibrillation
- Bone fracture
Fosamax should be taken on an empty stomach with a full glass of water. After taking the medication, the patient should remain upright and should not lie down for at least 30 minutes.
Fosamax Warnings
Bisphosphonates, or equivalents of Fosamax, are strongly recommended for menopausal women but there may have been a lack of information about long-term use. It is intended to reverse and prevent bone loss but may have a negative impact on the ability to regenerate new bone and may actually increase the risk of bone tissue death and new fracture formation.
In 1997, the U.S. Food and Drug Administration issued a warning regarding Merck’s marketing program of Fosamax, stating that the company had used misleading and false statements in advertising and requiring that certain labeling changes be made. In 2004, the FDA warned that drugs in the bisphosphonate family may lead to “dead jaw syndrome” or osteonecrosis of the jaw but no action was taken at the time.
In 2008, after numerous reports had been received regarding femur fracture, the U.S. Food and Drug Administration pressured Merck, the manufacturer of Fosamax, to address the reports. In 2009, Merck complied by adding information about the possibility of femur fracture to a list of potential side effects.
Unfortunately, there may have been little else done to inform the medical community or public about the risks. Patients may have continued to take the medication without full knowledge of the risks or symptoms that should be reported.
Studies published in the Journal of Orthopedic Trauma and the Journal of Bone and Mineral Research link Fosamax and other bisphosphonate drugs to a rare type of spontaneous fracture in the femur. Results showed those who were taking Fosamax on average for four years or more and had similar numbers of low-impact femur fractures.
In 2010, the FDA began working closely with experts from the American Society of Bone and Mineral Research Subtrochanteric Femoral Fracture Task Force, to gather additional information that promised to provide insight into the grave risks associated with the drug. As a result, updated FDA recommendations for healthcare professionals as well as patients about the possible risks and side effects of oral bisphosphonates based on mounting results of clinical trials and ongoing studies were published and are available on the FDA website.
Fosamax Settlements and Lawsuits
More than 4,000 lawsuits have been filed against Merck for injuries caused by Fosamax. Federal cases were consolidated into multidistrict litigation (MDL) in New Jersey and New York. The company had offered settlements to several patients for amounts ranging from $285,000 to $1.5 million, but many lawsuits were not settled at this time.
In June of 2018, the U.S. Supreme Court agreed to hear Merck & Co’s appeal over Fosamax lawsuits. The company had claimed that they were not liable for failing to warn the public because the FDA initially rejected proposed language for a warning. Lawyers for the plaintiffs argued that the FDA had only objected to the way the warning was worded but later approved a properly worded warning, and, in fact, required that newly written warning appear on all prescribing information.
Merck attempted multiple times to have lawsuits thrown out and finally prevailed. The appeal was heard by the U.S. Supreme Court and 500 cases of Fosamax Femur fracture lawsuits in New Jersey were dismissed in March 2022. The dismissal was based on the argument that federal law overrode state-based drug injury claims. Some Fosamax lawsuits have yet to be settled and remain in court systems.
Merck continues to face lawsuits for osteonecrosis developments in many patients. Federal Lawsuits for Fosamax-related lawsuits were consolidated into multidistrict litigation (MDL 1789) in the Southern District of New York. The company continues to fight approximately 1200 claims.